MIM Software Setup Instructions
Follow these step-by-step instructions to set up MIM software on your computer, or download the MIM Setup Guide.
Note: Due to rapid advances in and frequent updates to software, your version of MIM may look different. However, the tools and steps remain the same.
-
Please click on a link below to download the appropriate installer for the MIM Referring software.
For PC/Windows users: https://downloads.mimsoftware.com/MIM7.0.6_buildKB13-05.exe
For Mac users: https://downloads.mimsoftware.com/MIM7.0.6_buildKB13-05.dmg -
Install the application.
-
For PC/Windows users: click Run as Administrator to begin the download and follow the installation instructions; see screenshot below.
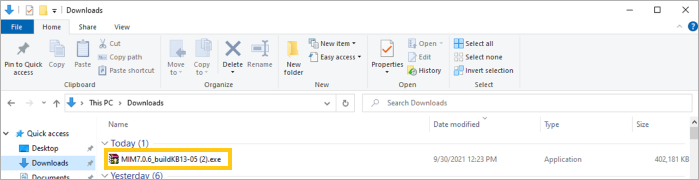
-
For Mac users: click on the downloaded .dmg file and then click on the PKG file to begin the installation.
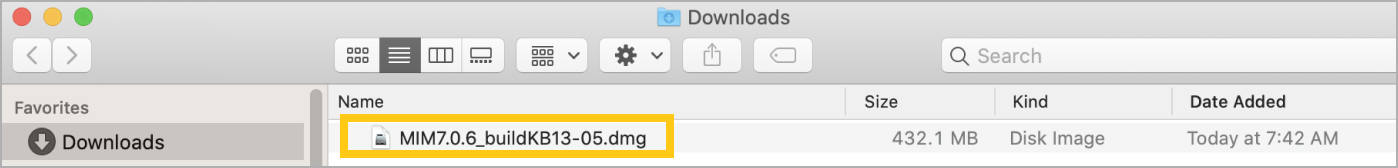
-
-
Enter the password korey (all lowercase) when prompted; see screenshot below.
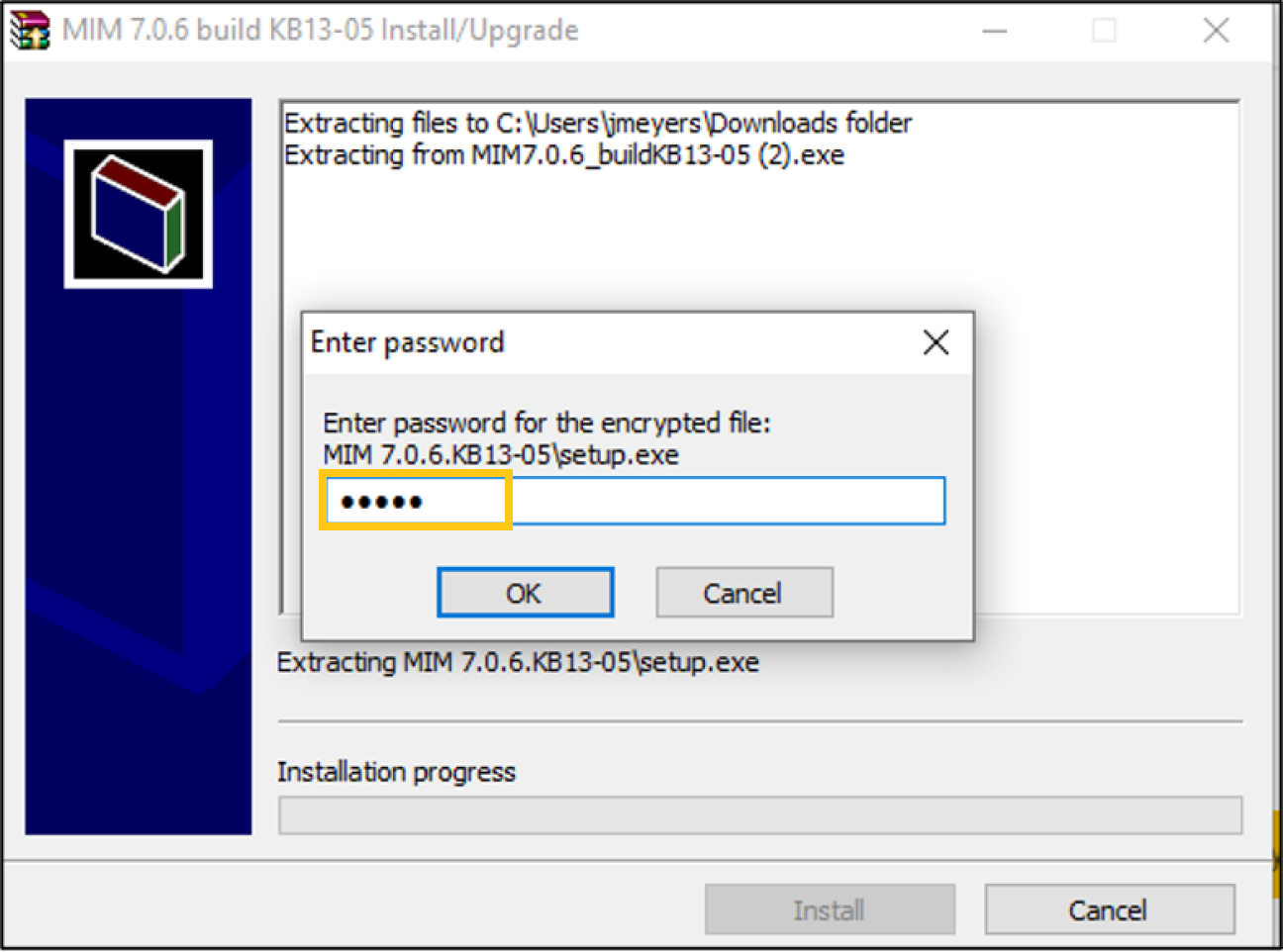
-
Once the installation completes, you will need to accept the end user license agreement and click Next; see screenshot below.
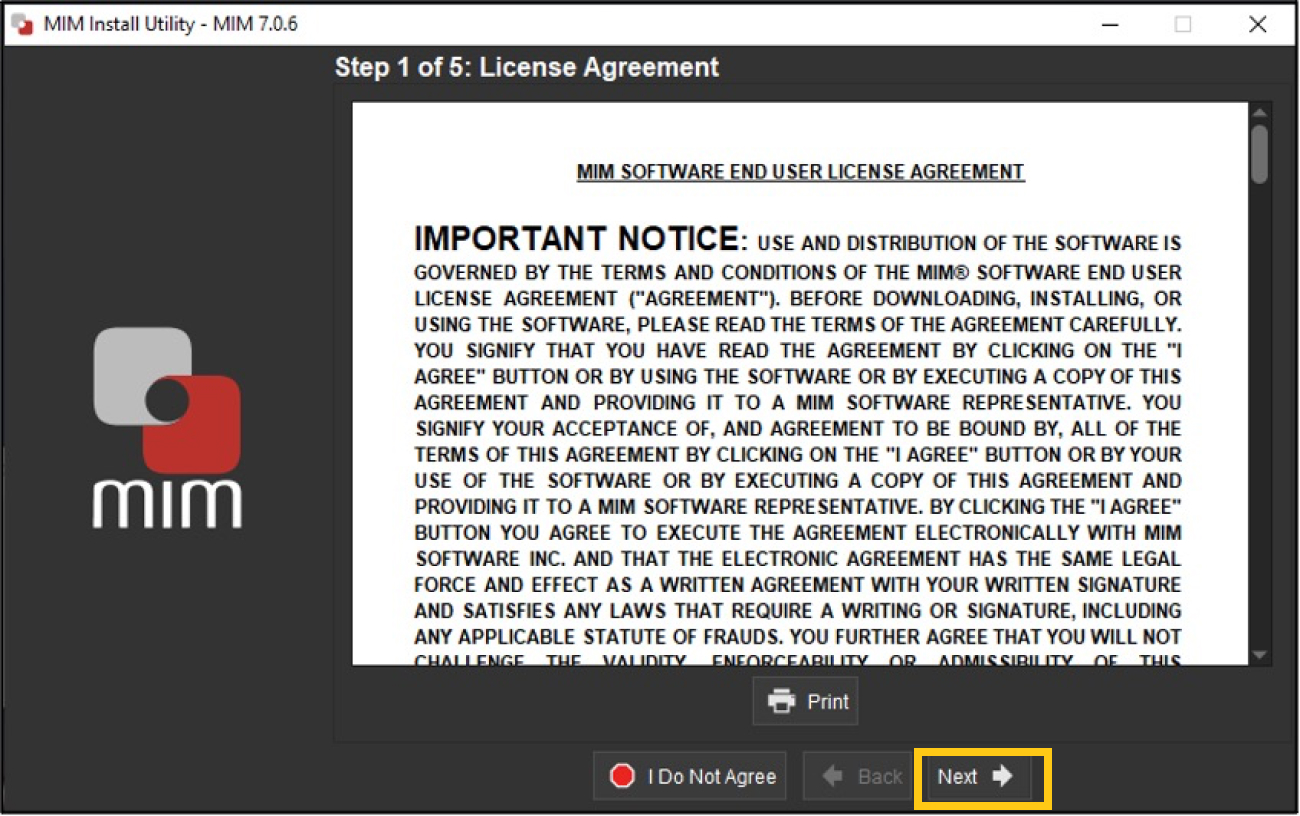
-
To configure the install, you can accept the file install location default choice or change it if desired; then click Next.
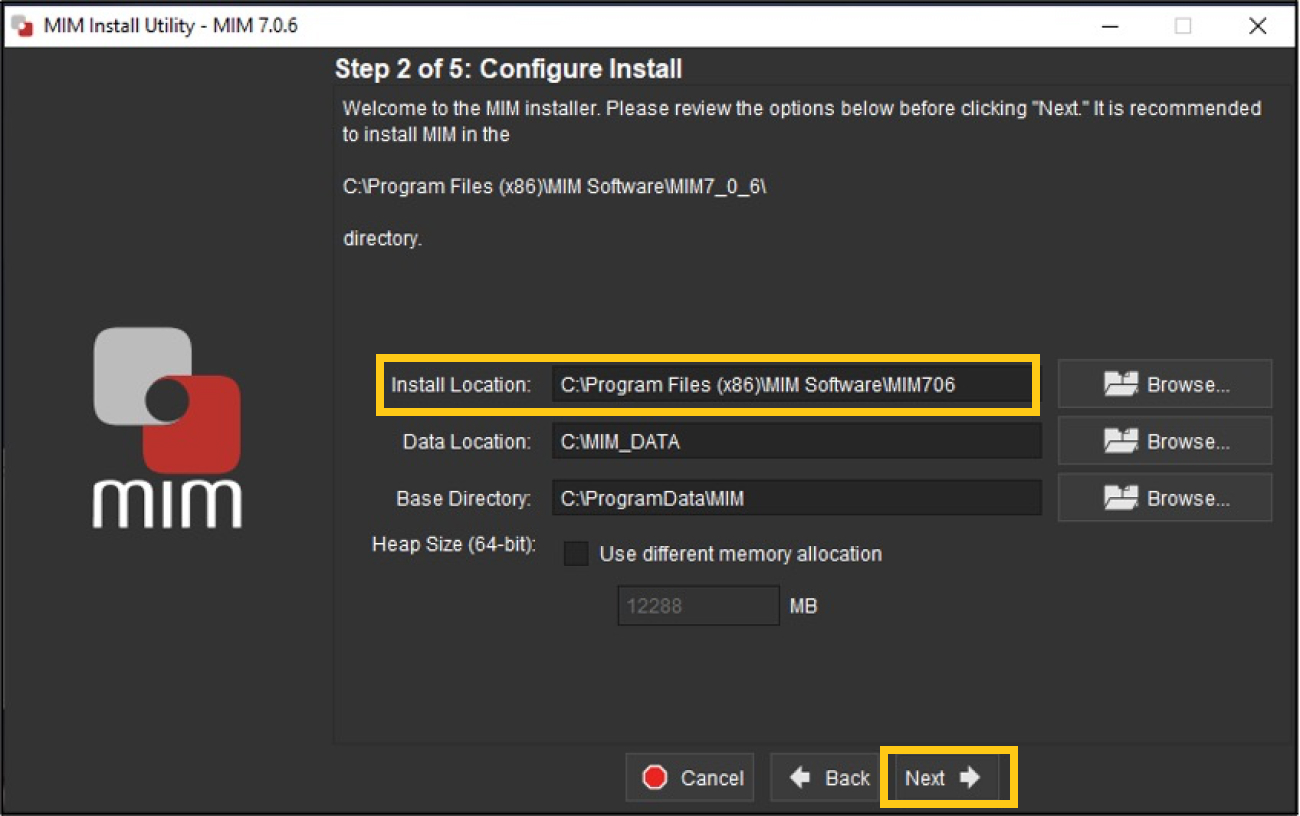
-
The next step asks if you’d like to create a shortcut; leave the selection at “All Users” and click Install; see screenshot below.
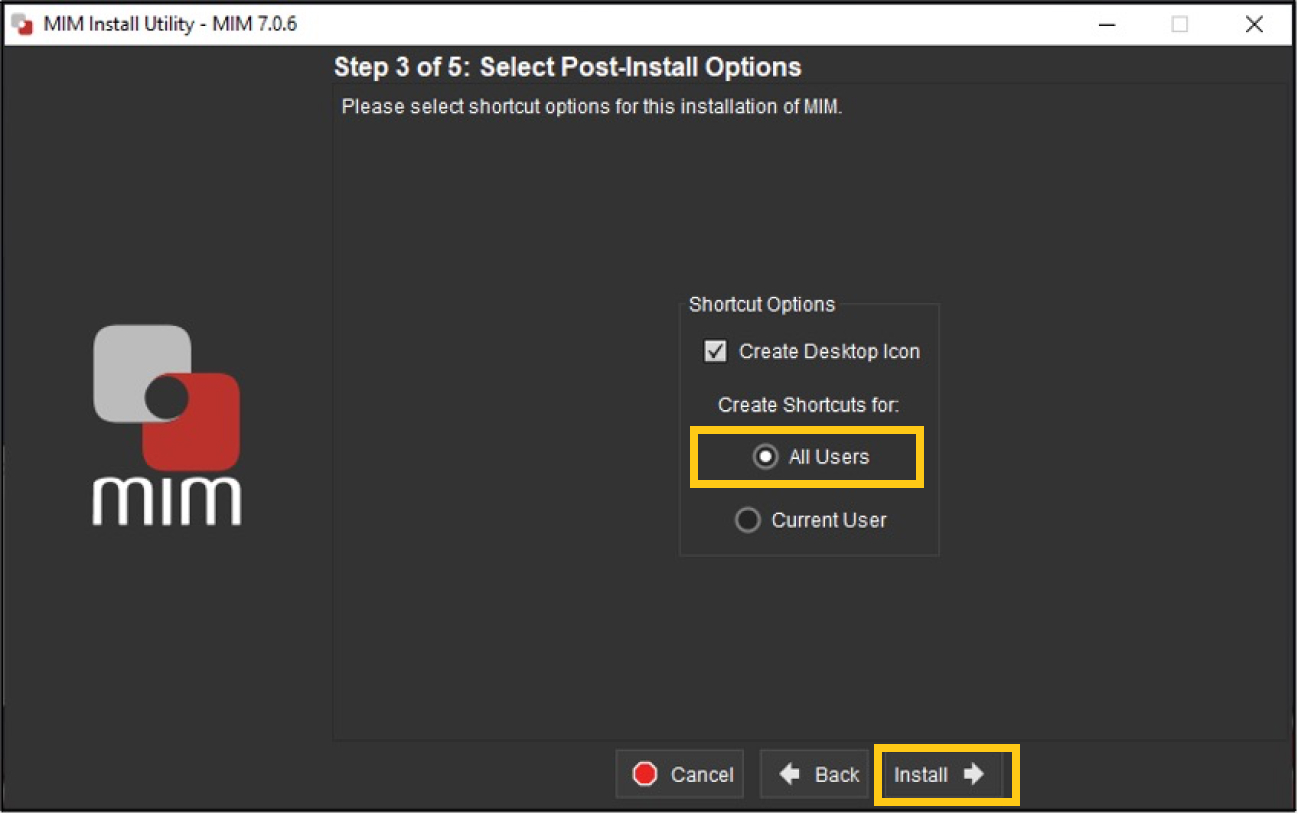
-
MIM will install.
-
Once finished installing, MIM will load after you click “Start MIM.”
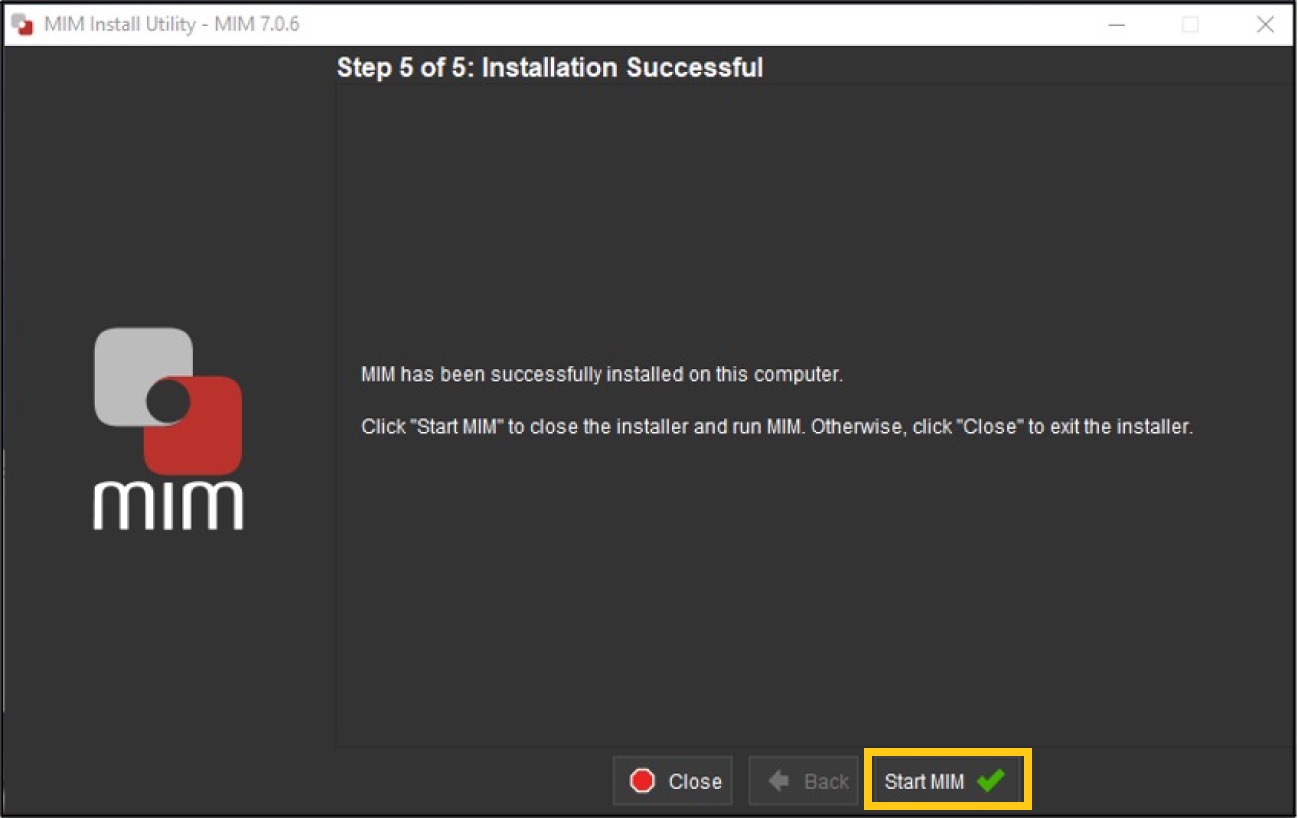
-
For the licensing option, select the top drop-down menu and switch from “Fixed” to “MIMCloud License” and enter the log-in credentials provided below.
- Username: TAUVID@TAUVIDReaderTraining.com
- Password: Tauvid2021
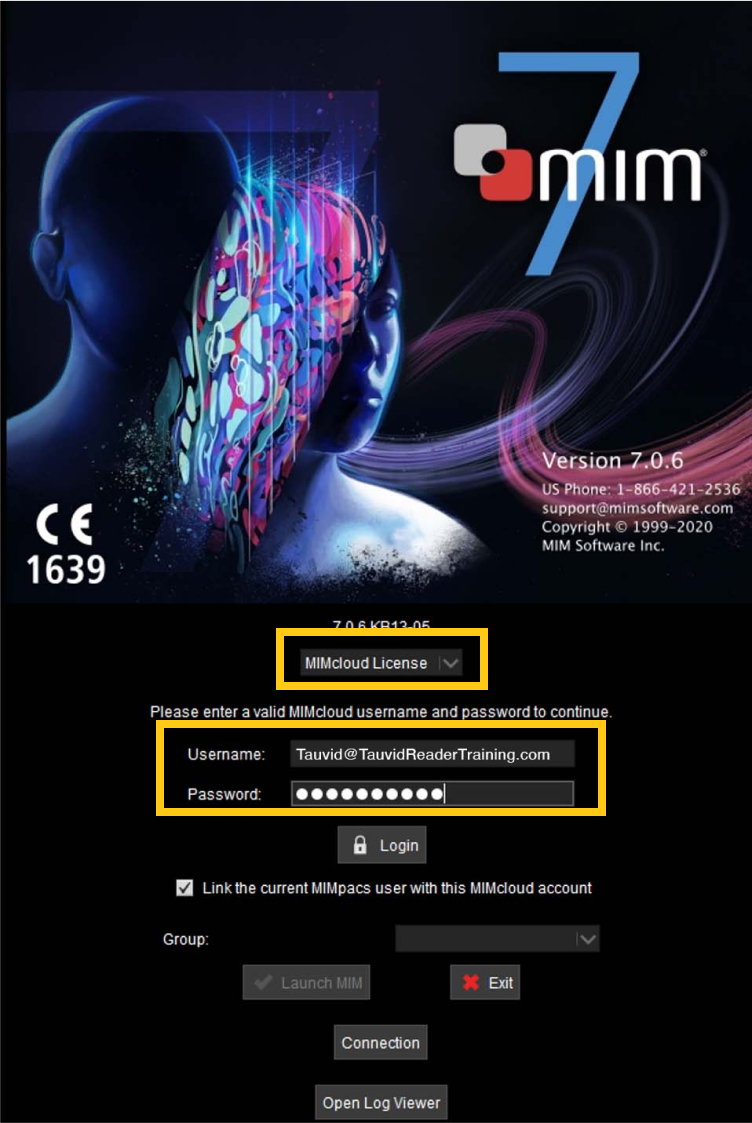
-
After entering the username and password listed in Step 9, select “MIM Referring” as your License Selection; then click OK to continue
